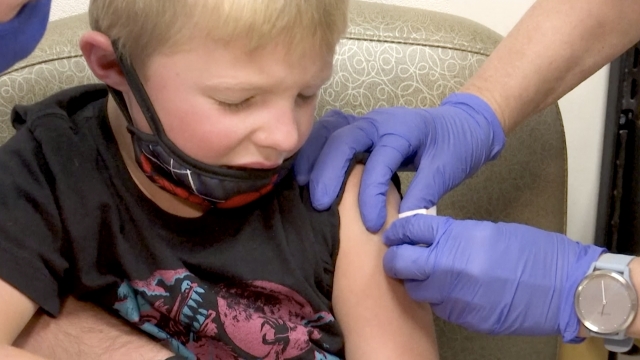
For most kids, getting vaccinated is nothing new. But for kids like Russell Bright the shots they’re getting now are.
"I know. That's why I've been trying to do all this stuff – so I can see my friends more," he said.
The 7-year-old and his 5-year-old brother Tucker are two of the more than 100 American kids participating in Pfizer's global COVID vaccine trial.
"When the opportunity presented itself, we wanted to get our kids enrolled in the study, you know, help make sure it's safe for other kids, as well as potentially have them vaccinated as quick as possible as well," Adam Bright, whose 2 sons are in the trial, said.
The trial includes children from 6 months to 11 years, in phases.
"So, the one that we started right now is 5 to 11, then we'll do 2 to 4, and then we'll do the six months to two years of age.” Julia Garcia-Diaz, director of global infectious disease research at Ochsner Medical Center, said.
Like other COVID-19 vaccine trials, these kids get their blood drawn, a nasal swab test, the vaccine and a diary to log any reaction or lack thereof. The only difference? These younger children will get lower doses than older ones or adults.
Less than 10% of confirmed COVID cases were in children, but the CDC says vaccinating them is vital.
"It not only helps him but the entire community - anyone vulnerable around our family. So, that's a great lesson to impart to our children," said Jason Halperin, whose son is in the trial.
If trial results prove they’re safe and effective, Pfizer is expected to request federal authorization in the fall for children as young as five.
Moderna and Johnson and Johnson are also testing their shots in children.