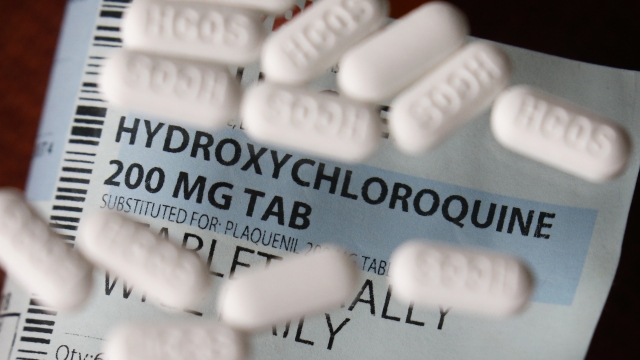
The National Institutes of Health has begun testing hydroxychloroquine as a potential treatment for the coronavirus.
The NIH said Thursday it's conducting a clinical trial to assess the antimalarial drug's "safety and effectiveness" on COVID-19 patients. Study participants will be adults hospitalized with COVID-19. They'll receive either hydroxychloroquine or a placebo twice daily for five days.
President Donald Trump has repeatedly touted the medication as a potential treatment, even going so far as encouraging people to "try it", despite little existing evidence of the drug's effectiveness in treating the coronavirus.
The NIH says some studies have shown the drug "demonstrated antiviral activity, [and] an ability to modify the activity of the immune system." But it also notes that even short-term use of hydroxychloroquine can cause heart issues, seizures and hypoglycemia.
Contains footage from CNN.